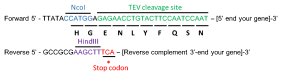Cloning of S100 with TEV cleavable 6xHis-tag (6xHis-TEV-S100):
For this cloning scheme, we begin with the MBP-LIC vector used for LIC cloning of MBP-fused S100 as described on another page.
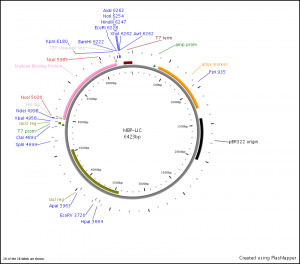
The MBP-LIC vector, shown below, contains maltose binding protein (MBP) with an N-terminal His-tag and a C-terminal TEV (tobacco etch virus protease) cleavage site (shown below). For this cloning reaction we wish to remove maltose binding protein from the vector, insert our S100 gene with the 6xHis-tag, followed by a TEV cleavage site. This will allow us to use Ni-affinity chromatography as our first step in purification of the S100, but remove the metal binding histidine tag prior to our biochemical experiments.
There is an NcoI restriction enzyme cleavage site immediately following the 6x-His-tag in the MBP-LIC vector. Using this NcoI site, if we insert our gene in frame, we can attach this His-tag to the N-terminus of the S100.
MBP-LIC vector 6xHis-Tag (4999-5024):
Using PCR, this NcoI site will be appended in the 5’ end of the S100 and a HindIII site will be appended to the 3’ end. As the HindIII site is present in the multiple cloning site of the vector this will allow for insertion of the S100 in the ORF currently occupied by MBP.
*Before proceeding, confirm that there are no additional NcoI and HindIII cleavage sites in the gene you plan to clone.
In order to make the histidine-tag removable with a TEV digestion, we need to insert the TEV cleavage site (E-N-L-Y-F-Q-S) between the 6xHis-tag and the start codon of the S100 gene. This is also done with PCR.
To create an insert for this cloning scheme, you must design primer which anneal to your gene of interest, the forward primer containing the NcoI site and TEV cleavage site in frame with your coding sequence, and the reverse primer containing a HindIII restriction site.
Primer design:
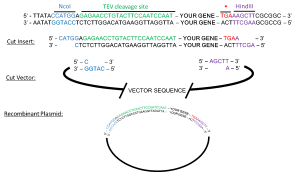
The resulting PCR product will contain restriction enzymes sites flanking the gene of interest. Next, we will digest the PCR product (insert) and MBP-LIC plasmid (vector) with NcoI and HindIII. These restriction enzymes cut leaving what are referred to as “sticky ends”. By purifying the cut insert and vector and then combining them in the presence of T4 DNA ligase, the sticky ends of the vector and insert will anneal, and the ligase will repair breaks in the DNA yielding the desired plasmid.
Recombinant Plasmid Construction:
Protocol:
1. Insert Amplification
The insert is amplified using a polymerase chain reaction. Two annealing temperatures are used. The first annealing temperature (Annealing Temp 1) should correspond to the melting temperature of the region of the primer which anneals to you gene of interest. The second annealing temperature (Annealing Temp 2) should correspond to the melting temperature of the complete primer.
Use the NEB Tm Calculator to find the annealing temperatures for your reaction with Q5 polymerase: https://www.neb.com/tools-and-resources/interactive-tools/tm-calculator
Prepare a PCR reaction with Q5 Polymerase:
| Reagent | Volume (µL) |
| Q5 Buffer (5X) | 5 µL |
| 100 mM dNTPs | 0.5 µL |
| 10 µM Fwd Primer | 1.25 µL |
| 10 µM Rev Primer | 1.25 µL |
| Template | 0.5 µL |
| H20 | 16.25 µL |
| Q5 | 0.25 µL |
| Total | 25 µL |
Use the “LIC-amp protocol” on the thermocycler, adjusting only the annealing temperatures to match those of your primer. Reaction protocol should start at 95oC for 15 sec, then 5 cycles of melt at 95oC (15 sec), anneal at Annealing Temp 1 (15 sec), and elongate at 72oC (30 sec) and then proceed with 30 cycles of melt at 95oC (15 sec), anneal at Annealing Temp 2 (15 sec), and elongate at 72oC (30 sec), and finish with a 2 min elongation step at 72oC.
Confirm PCR reaction was successful by analyzing 3-5 µL of the product on a 1% agarose gel.
2. Restriction Enzyme Digestion of Vector and Insert
Next we need to digest the vector and the insert with NcoI and HindIII.
We include DpnI in the insert digestion reaction. This enzyme cleaves methylated DNA, thereby removing any of our original PUC vector, used as template for the PCR reaction. This step proves particularly important if the plasmid we are amplifying from contains the same selection gene as the vector into which we are inserting our gene of interest.
Set up two independent digestions, one for the vector and another for the insert.
Vector Digestion (Digest 1-2 µg Vector DNA):
| Reagent | Volume (µL) |
| H2O | x µL |
| 10x Cut Smart Buffer | 2.5µL |
| MBP-LIC Vector | y µL |
| NcoI * | 0.5 µL |
| HindIII * | 0.5 µL |
| Total | 25 µL |
Insert Digestion:
| Reagent | Volume (µL) |
| H2O | 9 µL |
| 10x Cut Smart Buffer | 2.5 µL |
| PCR Product | 12 µL |
| NcoI * | 0.5 µL |
| HindIII * | 0.5 µL |
| DpnI * | 0.5 µL |
| Total | 25 µL |
Add enzymes last to reaction mixture. Mix entire reaction mixture gently by pipetting up and down with a P20 pipette. Incubate reaction mixtures on thermocycler at 37 C for 3 hr.
*Notes about proper use of restriction enzymes:
Enzyme stocks contain glycerol, they will settle to the bottom of the tube. Mix reaction mixtures gently by pipetting up and down following addition of enzymes. Take care to avoid contamination of enzyme stocks. Finally, enzymes must be kept cold, when pulling enzymes from the freezer, remove the entire freezer box, this box will keep the enzymes at optimal temperatures. Do not remove the boxes from the freezer until you are ready to use the enzymes, and promptly return enzymes to freezer when finished.
3. Purify Vector and Insert
Prepare a 1% agarose gel using large-well comb. Add 5 µL 6x DNA loading dye to vector digestion reaction mixture and load entire reaction mixture in 1 or 2 wells as necessary. Electrophorese for ~1 hr at 80 V. Keeping the gel on the plastic casing, visualize with UV transilluminator. Cut out the band of interest (~5200 bp) using razor blades and place gel fragment in a clean, massed, microfuge tube. Cut as close to the band of interest as possible, weight of gel fragment should be less than 200 mg.
Using the GeneJet Kit, purify vector DNA from gel fragment according to the manufacturer’s protocol.
Also, using the GeneJet Kit, following the protocol for purification of PCR reactions, purify the cut insert DNA.
4. Ligate with T4 DNA Ligase
| Reagent | Volume (µL) |
| H2O | 14.5 µL |
| 10x T4 DNA Ligase Buffer (NEB) | 2 µL |
| Vector | x µL |
| Insert | y µL |
| T4 DNA Ligase (NEB) | 1 µL |
| Total | 20 µL |
Vector and insert should be added in a 1:3 molar ratio. Given a 5200 bp vector fragment and a ~300 bp fragment, vector:insert mass ratios should be approximately 1:5. Use 20-50 ng of vector, and 4-10 ng insert in ligation reaction.
Once reaction mixture has been prepared either incubate 5 min at room temperature and overnight at 16oC. Or at room temperature for approximately 3 hr.
5. Transform
Using the manufacturer’s protocol for XL10-Gold Ultracompetent Cells, transform 2 µL ligation reaction mixture into 25 µL XL10-Gold Ultracompetent Cells. Plate cells following transformation onto LB-Amp Plates. Use sterile technique when working with bacterial cultures.
6. Verify Success
If transformants grow on LB-Amp plates, select three colonies from plate and set up 5 mL LB-Amp overnight cultures. In the morning purify plasmid DNA from 3-5 mL overnight culture using the GeneJet Plasmid MiniPrep Kit.
Using the gene-specific primers you designed, perform a PCR reaction on the plasmids to confirm that the gene has been inserted in the plasmid.
PCR Reaction:
| Reagent | Volume (µL) |
| Q5 Buffer (5X) | 5 µL |
| 100 mM dNTPs | 0.5 µL |
| 10 µM Fwd Primer | 1.25 µL |
| 10 µM Rev Primer | 1.25 µL |
| Template | 0.5 µL |
| H20 | 16.25 µL |
| Q5 | 0.25 µL |
| Total | 25 µL |
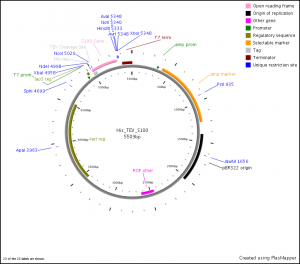
Additionally to confirm that the plasmid is the His_TEV_S100 plasmid, and that the gene is inserted in the right place in the vector, perform a restriction enzyme cleavage reaction using XbaI and HindIII. Use 0.5-1 µg DNA for the restriction digest. Incubate at 37oC for 1 hr. Prior to performing digest, confirm that your S100 gene does not contain XbaI sites or HindII sites.
Restriction Digest:
| Reagent | Volume (µL) |
| H2O | x µL |
| Recombinant Plasmid (0.5-1 µg) | y µL |
| HindIII | 0.5 µL |
| XbaI Remix | 0.5 µL |
| Total | 10 µL |
Visualize the product of the restriction digest and PCR reaction on a 1% agarose gel. Run 5 µL PCR product and total volume of restriction enzyme digest. PCR product should be ~300 bp in length, digested vector should yield bands of 5134 bp and 375 bp. If PCR product and restriction enzyme digest appear to be correct, choose one positive clone and submit for sequencing. T7 prom and T7 term primers can be used for sequencing.
>LIC_vector_MBP 6423 bp
tggcgaatgggacgcgccctgtagcggcgcattaagcgcggcgggtgtggtggttacgcg
cagcgtgaccgctacacttgccagcgccctagcgcccgctcctttcgctttcttcccttc
ctttctcgccacgttcgccggctttccccgtcaagctctaaatcgggggctccctttagg
gttccgatttagtgctttacggcacctcgaccccaaaaaacttgattagggtgatggttc
acattaacgcttacaatttaggtggcacttttcggggaaatgtgcgcggaacccctattt
gtttatttttctaaatacattcaaatatgtatccgctcatgagacaataaccctgataaa
tgcttcaataatttgaaaaaggaagagtatgagtattcaacatttccgtgtcgcccttat
tcccttttttgcggcattttgccttcctgtttttgctcacccagaaacgctggtgaaagt
aaaagatgctgaagatcagttgggtgcacgagtgggttacatcgaactggatctcaacag
cggtaagatccttgagagttttcgccccgaagaacgttttccaatgatgagcacttttaa
agttctgctatgtggcgcggtattatcccgtattgacgccgggcaagagcaactcggtcg
ccgcatacactattctcagaatgacttggttgagtactcaccagtcacagaaaagcatct
tacggatggcatgacagtaagagaattatgcagtgctgccataaccatgagtgataacac
tgcggccaacttacttctgacaacgatcggaggaccgaaggagctaaccgcttttttgca
caacatgggggatcatgtaactcgccttgatcgttgggaaccggagctgaatgaagccat
accaaacgacgagcgtgacaccacgatgcctgcagcaatggcaacaacgttgcgcaaact
attaactggcgaactacttactctagcttcccggcaacaattaatagactggatggaggc
ggataaagttgcaggaccacttctgcgctcggcccttccggctggctggtttattgctga
taaatctggagccggtgagcgtgggtctcgcggtatcattgcagcactggggccagatgg
taagccctcccgtatcgtagttatctacacgacggggagtcaggcaactatggatgaacg
aaatagacagatcgctgagataggtgcctcactgattaagcattggtaactgtcagacca
agtttactcatatatactttagattgatttaaaacttcatttttaatttaaaaggatcta
ggtgaagatcctttttgataatctcatgaccaaaatcccttaacgtgagttttcgttcca
ctgagcgtcagaccccgtagaaaagatcaaaggatcttcttgagatcctttttttctgcg
cgtaatctgctgcttgcaaacaaaaaaaccaccgctaccagcggtggtttgtttgccgga
tcaagagctaccaactctttttccgaaggtaactggcttcagcagagcgcagataccaaa
tactgtccttctagtgtagccgtagttaggccaccacttcaagaactctgtagcaccgcc
tacatacctcgctctgctaatcctgttaccagtggctgctgccagtggcgataagtcgtg
tcttaccgggttggactcaagacgatagttaccggataaggcgcagcggtcgggctgaac
ggggggttcgtgcacacagcccagcttggagcgaacgacctacaccgaactgagatacct
acagcgtgagctatgagaaagcgccacgcttcccgaagggagaaaggcggacaggtatcc
ggtaagcggcagggtcggaacaggagagcgcacgagggagcttccagggggaaacgcctg
gtatctttatagtcctgtcgggtttcgccacctctgacttgagcgtcgatttttgtgatg
ctcgtcaggggggcggagcctatggaaaaacgccagcaacgcggcctttttacggttcct
ggccttttgctggccttttgctcacatgttctttcctgcgttatcccctgattctgtgga
taaccgtattaccgcctttgagtgagctgataccgctcgccgcagccgaacgaccgagcg
cagcgagtcagtgagcgaggaagcggaagagcgcctgatgcggtattttctccttacgca
tctgtgcggtatttcacaccgcatatatggtgcactctcagtacaatctgctctgatgcc
gcatagttaagccagtatacactccgctatcgctacgtgactgggtcatggctgcgcccc
gacacccgccaacacccgctgacgcgccctgacgggcttgtctgctcccggcatccgctt
acagacaagctgtgaccgtctccgggagctgcatgtgtcagaggttttcaccgtcatcac
cgaaacgcgcgaggcagctgcggtaaagctcatcagcgtggtcgtgaagcgattcacaga
tgtctgcctgttcatccgcgtccagctcgttgagtttctccagaagcgttaatgtctggc
ttctgataaagcgggccatgttaagggcggttttttcctgtttggtcactgatgcctccg
tgtaagggggatttctgttcatgggggtaatgataccgatgaaacgagagaggatgctca
cgatacgggttactgatgatgaacatgcccggttactggaacgttgtgagggtaaacaac
tggcggtatggatgcggcgggaccagagaaaaatcactcagggtcaatgccagcgcttcg
ttaatacagatgtaggtgttccacagggtagccagcagcatcctgcgatgcagatccgga
acataatggtgcagggcgctgacttccgcgtttccagactttacgaaacacggaaaccga
agaccattcatgttgttgctcaggtcgcagacgttttgcagcagcagtcgcttcacgttc
gctcgcgtatcggtgattcattctgctaaccagtaaggcaaccccgccagcctagccggg
tcctcaacgacaggagcacgatcatgcgcacccgtggggccgccatgccggcgataatgg
cctgcttctcgccgaaacgtttggtggcgggaccagtgacgaaggcttgagcgagggcgt
gcaagattccgaataccgcaagcgacaggccgatcatcgtcgcgctccagcgaaagcggt
cctcgccgaaaatgacccagagcgctgccggcacctgtcctacgagttgcatgataaaga
agacagtcataagtgcggcgacgatagtcatgccccgcgcccaccggaaggagctgactg
ggttgaaggctctcaagggcatcggtcgagatcccggtgcctaatgagtgagctaactta
cattaattgcgttgcgctcactgcccgctttccagtcgggaaacctgtcgtgccagctgc
attaatgaatcggccaacgcgcggggagaggcggtttgcgtattgggcgccagggtggtt
tttcttttcaccagtgagacgggcaacagctgattgcccttcaccgcctggccctgagag
agttgcagcaagcggtccacgctggtttgccccagcaggcgaaaatcctgtttgatggtg
gttaacggcgggatataacatgagctgtcttcggtatcgtcgtatcccactaccgagata
tccgcaccaacgcgcagcccggactcggtaatggcgcgcattgcgcccagcgccatctga
tcgttggcaaccagcatcgcagtgggaacgatgccctcattcagcatttgcatggtttgt
tgaaaaccggacatggcactccagtcgccttcccgttccgctatcggctgaatttgattg
cgagtgagatatttatgccagccagccagacgcagacgcgccgagacagaacttaatggg
cccgctaacagcgcgatttgctggtgacccaatgcgaccagatgctccacgcccagtcgc
gtaccgtcttcatgggagaaaataatactgttgatgggtgtctggtcagagacatcaaga
aataacgccggaacattagtgcaggcagcttccacagcaatggcatcctggtcatccagc
ggatagttaatgatcagcccactgacgcgttgcgcgagaagattgtgcaccgccgcttta
caggcttcgacgccgcttcgttctaccatcgacaccaccacgctggcacccagttgatcg
gcgcgagatttaatcgccgcgacaatttgcgacggcgcgtgcagggccagactggaggtg
gcaacgccaatcagcaacgactgtttgcccgccagttgttgtgccacgcggttgggaatg
taattcagctccgccatcgccgcttccactttttcccgcgttttcgcagaaacgtggctg
gcctggttcaccacgcgggaaacggtctgataagagacaccggcatactctgcgacatcg
tataacgttactggtttcacattcaccaccctgaattgactctcttccgggcgctatcat
gccataccgcgaaaggttttgcgccattcgatggtgtccgggatctcgacgctctccctt
atgcgactcctgcattaggaagcagcccagtagtaggttgaggccgttgagcaccgccgc
cgcaaggaatggtgcatgcaaggagatggcgcccaacagtcccccggccacggggcctgc
caccatacccacgccgaaacaagcgctcatgagcccgaagtggcgagcccgatcttcccc
atcggtgatgtcggcgatataggcgccagcaaccgcacctgtggcgccggtgatgccggc
cacgatgcgtccggcgtagaggatcgagatcgatctcgatcccgcgaaattaatacgact
cactataggggaattgtgagcggataacaattcccctctagaaataattttgtttaactt
taagaaggagatatacatatgaaacaccatcaccatcaccatggcaaaatcgaagaaggt
aaactggtaatctggattaacggcgataaaggctataacggtctcgctgaagtcggtaag
aaattcgagaaagataccggaattaaagtcaccgttgagcatccggataaactggaagag
aaattcccacaggttgcggcaactggcgatggccctgacattatcttctgggcacacgac
cgctttggtggctacgctcaatctggcctgttggctgaaatcaccccggacaaagcgttc
caggacaagctgtatccgtttacctgggatgccgtacgttacaacggcaagctgattgct
tacccgatcgctgttgaagcgttatcgctgatttataacaaagatctgctgccgaacccg
ccaaaaacctgggaagagatcccggcgctggataaagaactgaaagcgaaaggtaagagc
gcgctgatgttcaacctgcaagaaccgtacttcacctggccgctgattgctgctgacggg
ggttatgcgttcaagtatgaaaacggcaagtacgacattaaagacgtgggcgtggataac
gctggcgcgaaagcgggtctgaccttcctggttgacctgattaaaaacaaacacatgaat
gcagacaccgattactccatcgcagaagctgcctttaataaaggcgaaacagcgatgacc
atcaacggcccgtgggcatggtccaacatcgacaccagcaaagtgaattatggtgtaacg
gtactgccgaccttcaagggtcaaccatccaaaccgttcgttggcgtgctgagcgcaggt
attaacgccgccagtccgaacaaagagctggcaaaagagttcctcgaaaactatctgctg
actgatgaaggtctggaagcggttaataaagacaaaccgctgggtgccgtagcgctgaag
tcttacgaggaagagttggcgaaagatccacgtattgccgccaccatggaaaacgcccag
aaaggtgaaatcatgccgaacatcccgcagatgtccgctttctggtatgccgtgcgtact
gcggtgatcaacgccgccagcggtcgtcagactgtcgatgaagccctgaaagacgcgcag
actaattcgagctcgaacaacaacaacaataacaataacaacaacagagatctgggtacc
gagaacctgtacttccaatccaatattggaagtggataacggatccgaattcgagctccg
tcgacaagcttgcggccgcactcgagcaccaccaccaccaccactgagatccggctgcta
acaaagcccgaaaggaagctgagttggctgctgccaccgctgagcaataactagcataac
cccttggggcctctaaacgggtcttgaggggttttttgctgaaaggaggaactatatccg
gat
>His_TEV_S100A8
tggcgaatgggacgcgccctgtagcggcgcattaagcgcggcgggtgtggtggttacgcg
cagcgtgaccgctacacttgccagcgccctagcgcccgctcctttcgctttcttcccttc
ctttctcgccacgttcgccggctttccccgtcaagctctaaatcgggggctccctttagg
gttccgatttagtgctttacggcacctcgaccccaaaaaacttgattagggtgatggttc
acattaacgcttacaatttaggtggcacttttcggggaaatgtgcgcggaacccctattt
gtttatttttctaaatacattcaaatatgtatccgctcatgagacaataaccctgataaa
tgcttcaataatttgaaaaaggaagagtatgagtattcaacatttccgtgtcgcccttat
tcccttttttgcggcattttgccttcctgtttttgctcacccagaaacgctggtgaaagt
aaaagatgctgaagatcagttgggtgcacgagtgggttacatcgaactggatctcaacag
cggtaagatccttgagagttttcgccccgaagaacgttttccaatgatgagcacttttaa
agttctgctatgtggcgcggtattatcccgtattgacgccgggcaagagcaactcggtcg
ccgcatacactattctcagaatgacttggttgagtactcaccagtcacagaaaagcatct
tacggatggcatgacagtaagagaattatgcagtgctgccataaccatgagtgataacac
tgcggccaacttacttctgacaacgatcggaggaccgaaggagctaaccgcttttttgca
caacatgggggatcatgtaactcgccttgatcgttgggaaccggagctgaatgaagccat
accaaacgacgagcgtgacaccacgatgcctgcagcaatggcaacaacgttgcgcaaact
attaactggcgaactacttactctagcttcccggcaacaattaatagactggatggaggc
ggataaagttgcaggaccacttctgcgctcggcccttccggctggctggtttattgctga
taaatctggagccggtgagcgtgggtctcgcggtatcattgcagcactggggccagatgg
taagccctcccgtatcgtagttatctacacgacggggagtcaggcaactatggatgaacg
aaatagacagatcgctgagataggtgcctcactgattaagcattggtaactgtcagacca
agtttactcatatatactttagattgatttaaaacttcatttttaatttaaaaggatcta
ggtgaagatcctttttgataatctcatgaccaaaatcccttaacgtgagttttcgttcca
ctgagcgtcagaccccgtagaaaagatcaaaggatcttcttgagatcctttttttctgcg
cgtaatctgctgcttgcaaacaaaaaaaccaccgctaccagcggtggtttgtttgccgga
tcaagagctaccaactctttttccgaaggtaactggcttcagcagagcgcagataccaaa
tactgtccttctagtgtagccgtagttaggccaccacttcaagaactctgtagcaccgcc
tacatacctcgctctgctaatcctgttaccagtggctgctgccagtggcgataagtcgtg
tcttaccgggttggactcaagacgatagttaccggataaggcgcagcggtcgggctgaac
ggggggttcgtgcacacagcccagcttggagcgaacgacctacaccgaactgagatacct
acagcgtgagctatgagaaagcgccacgcttcccgaagggagaaaggcggacaggtatcc
ggtaagcggcagggtcggaacaggagagcgcacgagggagcttccagggggaaacgcctg
gtatctttatagtcctgtcgggtttcgccacctctgacttgagcgtcgatttttgtgatg
ctcgtcaggggggcggagcctatggaaaaacgccagcaacgcggcctttttacggttcct
ggccttttgctggccttttgctcacatgttctttcctgcgttatcccctgattctgtgga
taaccgtattaccgcctttgagtgagctgataccgctcgccgcagccgaacgaccgagcg
cagcgagtcagtgagcgaggaagcggaagagcgcctgatgcggtattttctccttacgca
tctgtgcggtatttcacaccgcatatatggtgcactctcagtacaatctgctctgatgcc
gcatagttaagccagtatacactccgctatcgctacgtgactgggtcatggctgcgcccc
gacacccgccaacacccgctgacgcgccctgacgggcttgtctgctcccggcatccgctt
acagacaagctgtgaccgtctccgggagctgcatgtgtcagaggttttcaccgtcatcac
cgaaacgcgcgaggcagctgcggtaaagctcatcagcgtggtcgtgaagcgattcacaga
tgtctgcctgttcatccgcgtccagctcgttgagtttctccagaagcgttaatgtctggc
ttctgataaagcgggccatgttaagggcggttttttcctgtttggtcactgatgcctccg
tgtaagggggatttctgttcatgggggtaatgataccgatgaaacgagagaggatgctca
cgatacgggttactgatgatgaacatgcccggttactggaacgttgtgagggtaaacaac
tggcggtatggatgcggcgggaccagagaaaaatcactcagggtcaatgccagcgcttcg
ttaatacagatgtaggtgttccacagggtagccagcagcatcctgcgatgcagatccgga
acataatggtgcagggcgctgacttccgcgtttccagactttacgaaacacggaaaccga
agaccattcatgttgttgctcaggtcgcagacgttttgcagcagcagtcgcttcacgttc
gctcgcgtatcggtgattcattctgctaaccagtaaggcaaccccgccagcctagccggg
tcctcaacgacaggagcacgatcatgcgcacccgtggggccgccatgccggcgataatgg
cctgcttctcgccgaaacgtttggtggcgggaccagtgacgaaggcttgagcgagggcgt
gcaagattccgaataccgcaagcgacaggccgatcatcgtcgcgctccagcgaaagcggt
cctcgccgaaaatgacccagagcgctgccggcacctgtcctacgagttgcatgataaaga
agacagtcataagtgcggcgacgatagtcatgccccgcgcccaccggaaggagctgactg
ggttgaaggctctcaagggcatcggtcgagatcccggtgcctaatgagtgagctaactta
cattaattgcgttgcgctcactgcccgctttccagtcgggaaacctgtcgtgccagctgc
attaatgaatcggccaacgcgcggggagaggcggtttgcgtattgggcgccagggtggtt
tttcttttcaccagtgagacgggcaacagctgattgcccttcaccgcctggccctgagag
agttgcagcaagcggtccacgctggtttgccccagcaggcgaaaatcctgtttgatggtg
gttaacggcgggatataacatgagctgtcttcggtatcgtcgtatcccactaccgagata
tccgcaccaacgcgcagcccggactcggtaatggcgcgcattgcgcccagcgccatctga
tcgttggcaaccagcatcgcagtgggaacgatgccctcattcagcatttgcatggtttgt
tgaaaaccggacatggcactccagtcgccttcccgttccgctatcggctgaatttgattg
cgagtgagatatttatgccagccagccagacgcagacgcgccgagacagaacttaatggg
cccgctaacagcgcgatttgctggtgacccaatgcgaccagatgctccacgcccagtcgc
gtaccgtcttcatgggagaaaataatactgttgatgggtgtctggtcagagacatcaaga
aataacgccggaacattagtgcaggcagcttccacagcaatggcatcctggtcatccagc
ggatagttaatgatcagcccactgacgcgttgcgcgagaagattgtgcaccgccgcttta
caggcttcgacgccgcttcgttctaccatcgacaccaccacgctggcacccagttgatcg
gcgcgagatttaatcgccgcgacaatttgcgacggcgcgtgcagggccagactggaggtg
gcaacgccaatcagcaacgactgtttgcccgccagttgttgtgccacgcggttgggaatg
taattcagctccgccatcgccgcttccactttttcccgcgttttcgcagaaacgtggctg
gcctggttcaccacgcgggaaacggtctgataagagacaccggcatactctgcgacatcg
tataacgttactggtttcacattcaccaccctgaattgactctcttccgggcgctatcat
gccataccgcgaaaggttttgcgccattcgatggtgtccgggatctcgacgctctccctt
atgcgactcctgcattaggaagcagcccagtagtaggttgaggccgttgagcaccgccgc
cgcaaggaatggtgcatgcaaggagatggcgcccaacagtcccccggccacggggcctgc
caccatacccacgccgaaacaagcgctcatgagcccgaagtggcgagcccgatcttcccc
atcggtgatgtcggcgatataggcgccagcaaccgcacctgtggcgccggtgatgccggc
cacgatgcgtccggcgtagaggatcgagatcgatctcgatcccgcgaaattaatacgact
cactataggggaattgtgagcggataacaattcccctctagaaataattttgtttaactt
taagaaggagatatacatatgaaacaccatcaccatcaCCATGGAGAGAACCTGTACTTC
CAATCCAATATGCTGACCGAACTGGAAAAAGCCCTGAACTCAATTATCGATGTCTACCAC
AAATACTCGCTGATTAAAGGCAACTTCCACGCTGTTTATCGTGATGACCTGAAAAAACTG
CTGGAAACCGAATGCCCGCAGTACATTCGCAAAAAAGGCGCAGATGTCTGGTTTAAAGAA
CTGGATATCAACACGGACGGTGCGGTTAACTTCCAAGAATTTCTGATCCTGGTGATCAAA
ATGGGCGTTGCGGCCCATAAAAAATCTCACGAAGAATCTCATAAAGAATGAAAGCTTgcg
gccgcactcgagcaccaccaccaccaccactgagatccggctgctaacaaagcccgaaag
gaagctgagttggctgctgccaccgctgagcaataactagcataaccccttggggcctct
aaacgggtcttgaggggttttttgctgaaaggaggaactatatccggat